 What is the ATLANTIS Study?
What is the ATLANTIS Study?
Thank you for your interest in the ATLANTIS Study. The ATLANTIS Study is a multinational clinical research study testing the study medication, amlitelimab, in children and adults 12 years of age and older with moderate to severe atopic dermatitis.
Researchers want to see if the study medication (amlitelimab) can safely treat moderate to severe atopic dermatitis over a long period of time.
Amlitelimab is not yet approved by health authorities such as the US Food and Drug Administration (FDA) and is still under investigation for the treatment of atopic dermatitis, so its effectiveness and safety have not been established.
Click below to download resources containing more information on atopic dermatitis and the study.
Understanding Clinical Trials (for 12 to 17 year olds)
Mire el video para saber más sobre los estudios de investigación clínica.
Understanding Atopic Dermatitis (for 12 to 17 year olds)
Mire el video para saber más sobre la dermatitis atópica.

 Who can participate in the ATLANTIS Study?
Who can participate in the ATLANTIS Study?
This study is for children and adults 12 years of age and older:
- Who have had moderate to severe atopic dermatitis for 1 year or longer.
- For whom topical medication is not advised OR
- Whose symptoms did not improve with topical medication in the last 6 months.
También se aplican otros criterios de participación.
 What study medication will I receive?
What study medication will I receive?
If you qualify for the study, you will receive the study medication, amlitelimab, every 4 weeks for about 3 years. You will receive your final dose at Week 156.
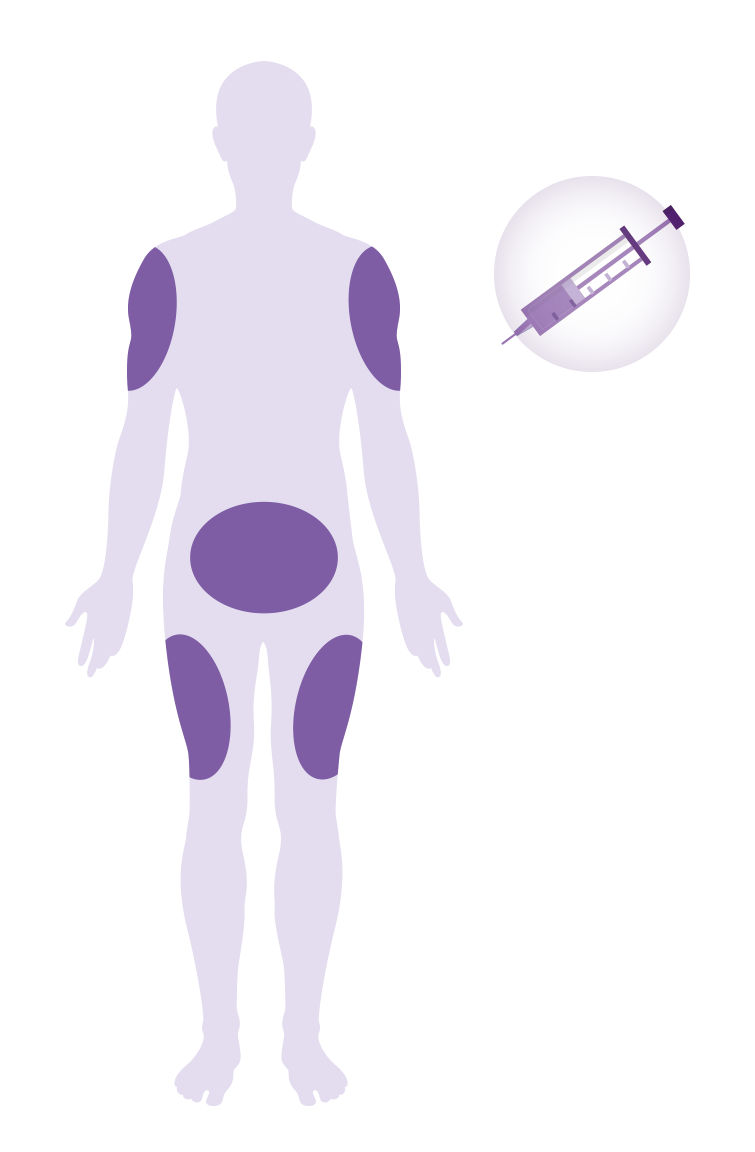
Amlitelimab is given as an injection (shot) into the fatty layer just beneath the skin. It can be given in the abdomen, outer thigh, or upper arm.*
- For the first year (52 weeks) you will receive your dose of amlitelimab at the study site.
- After that, you may choose to continue receiving your injections at the study site or you/your caregiver can be trained to give the injection at home.
During the study you may be able to keep using certain topical medications, moisturizers, and steroid creams. The study doctor will let you know which treatments you are allowed to use.
*Upper arms can only be injected by a caregiver, not by the participant him/herself.
 What happens during the study?
What happens during the study?
First you will need to give your consent, or permission, to join the study before any study procedures can begin by reading and signing an Informed Consent Form. Participants who are 12 to 17 years of age will sign a similar form called the Informed Assent Form in addition to the Informed Consent Form signed by the participant’s legal authorized representative.
El estudio ATLANTIS tiene una duración de unos 3 años y medio y se divide en 3 partes. Mientras esté en el estudio, asistirá a unas 26 visitas para procedimientos y revisiones de salud.
Screening Period
(dura de 2 a 4 semanas)
To determine whether you qualify for the study, the study team will ask questions about your health and the medicines you take and will run some tests.
Periodo de tratamiento del estudio
Dura 160 semanas (unos 3 años)
Recibirá el medicamento del estudio, amlitelimab, cada 4 semanas durante unos 3 años (160 semanas). Recibirá su dosis final en la Semana 156. Durante el primer año asistirá a visitas en las Semanas 0, 2 y 4, y posteriormente cada 4 semanas hasta la Semana 52. Durante los 2 años siguientes (aproximadamente), asistirá a una visita cada 3 meses (12 semanas).
Periodo de seguimiento
20 semanas después de la dosis final del PEI (unos 5 meses)
20 semanas después de su dosis final de amlitelimab, asistirá a 1 visita más para revisar su estado de salud.
 What kind of tests and health checks will I have?
What kind of tests and health checks will I have?
During the study you will have about 26 on-site visits for tests and health checks. You will not have all these tests at each visit. There may also be additional tests. Talk to the study doctor for more information.
Examen físico
Estatura
Peso
Signos vitales
Actividad cardíaca (ECG)
Análisis de sangre
Análisis de orina
Prueba de COVID-19
Puntuación de gravedad de la dermatitis atópica
Cuestionarios
Revisión de efectos secundarios
Mental health check
¿Hay alguna otra cosa que tendré que hacer durante el estudio?
You will have an electronic diary (eDiary) app installed on your phone or on a device provided to you. The study team will train you on how to use this app to complete questionnaires. You’ll complete questionnaires every day for the first 6 months, then at different times during the remainder of the study. The study team will let you know when you are expected to complete the questionnaires.